
ArgenxSE has opened up a new and potentially fruitful age of drug discovery.
Some autoimmune diseases are driven by immunoglobulin G (IgG) antibodies that mistakenly attack different parts of the body. Thanks to an emerging area of science, a new class of medicines may help advance the treatment landscape for many of these diseases, and improve symptoms for numerous people. The approach revolves around a breakthrough discovery about the neonatal Fc receptor (also known as FcRn) and its role in recycling IgG antibodies.
FcRn – shorthand for “neonatal Fc receptor” – is a protein and Fc receptor found in several types of tissues and cells in the body. It plays a role at two different phases of our lives:
In early development, FcRn is responsible for passing immunity from mother to child
Over a person’s lifetime, FcRn helps maintain the number of antibodies present in a person’s body.
FcRn recycles some of the immune system’s frontline forces – antibodies called IgGs. These antibodies have binding sites that fit with FcRn receptors, allowing them to avoid degradation. When an IgG antibody connects to an FcRn receptor, it gets recycled back into the bloodstream and stays in circulation. Unfortunately, the IgG recycling functionality of FcRn may also play a major role in many autoimmune diseases.
In the late 1990s, Prof. Sally Ward and her team at the University of Texas Southwestern Medical Center identified the way FcRn receptors bind to IgG antibodies and recycle them into the blood. Years later, Ward and the argenx team began collaborating to find a way to block IgG recycling – hypothesizing that it could mitigate the symptoms of some IgG-mediated autoimmune disorders.
An FcRn blocker works by binding to FcRn receptors and blocking them, resulting in fewer IgG and harmful IgG antibodies from attaching and getting recycled.
Examples of IgG-Mediated Autoimmune Diseases
gMG (Generalized Myasthenia Gravis) – Neurological. – Severe muscle weakness, including difficulty speaking, swallowing, and breathing.
ITP (Idiopathic Thrombocytopenic Purpura). – Hematology – Bruising and bleeding
PV (Pemphigus Vulgaris) – Dermatological – Blistering of the skin and mucous membranes.
CIDP (Chronic inflammatory Demyelinating Polyradiculoneuropathy) – Neurological – Weakness in the arms and legs
Bullous Pemphigoid – Dermatological – Large, fluid-filled blisters, often on areas of skin that flex, such as the lower abdomen, upper thighs, and armpits
Idiopathic Inflammatory Myositis – Neurological – Muscle inflammation and weakness
FcRn blockers are designed to block IgG recycling at the source, the FcRn receptor. Reducing IgG antibody circulation by blocking FcRn in autoimmune diseases is an area of ongoing research.
ArgenxSE on immunology innovation, 15 November 2025
There is so much happening at Argenx.
argenx is advancing its Vision 2030 strategic priorities, anchored in the ambition to treat 50,000 patients globally with its medicines, secure 10 labeled indications across approved medicines, and progress five pipeline candidates into Phase 3 development by 2030.
Q3 results and update, 30 October 2025
It already has one hugely successful drug with fast-growing sales in three indications so far.
VYVGART® (IV: efgartigimod alfa-fcab and SC: efgartigimod alfa and hyaluronidase-qvfc) is a first-and-only IgG Fc-antibody fragment that targets the neonatal Fc receptor (FcRn). It is approved in three indications, including generalized myasthenia gravis (gMG) and chronic inflammatory demyelinating polyneuropathy (CIDP) globally, and primary immune thrombocytopenia (ITP) in Japan.
Delivered $1.13 billion in global product net sales in the third quarter of 2025, an increase of $554 million year-over-year and $178 million quarter-over-quarter, reflecting strong fundamentals and continued confidence from patients and prescribers.
VYVGART SC prefilled syringe (PFS) for self-injection approved in Japan in September 2025; Canada decision on approval expected by end of 2025
Supplemental Biologics License Application (sBLA) for VYVGART in three anti-acetylcholine receptor antibody negative (AChR-Ab seronegative) gMG subtypes (MuSK+, LRP4+, triple seronegative) on track for filing with U.S. Food and Drug Administration (FDA) by year-end 2025
Pursuing label expansion through ongoing registrational studies:
Topline results expected in first half of 2026 for ocular MG (ADAPT OCULUS)
Topline results expected in the second half of 2026 for primary ITP (ADVANCE-NEXT)
Expanded partnership with FUJIFILM to include new manufacturing site in North Carolina, strengthening global supply chain and supporting anticipated growth in efgartigimod and pipeline assets
Q3 results and update, 30 October 2025
The pipeline looks incredibly strong.
argenx continues to demonstrate breadth and depth within its immunology pipeline, advancing multiple first-in-class product candidates with potential across high-need indications.
Efgartigimod Development
Efgartigimod is being studied in severe IgG-mediated autoimmune diseases, highlighting the broad potential of FcRn biology across several therapeutic areas including neurology, rheumatology and endocrinology.
Registrational studies are currently ongoing in two rheumatology indications, including idiopathic inflammatory myopathies (IIM or myositis) and Sjögren’s disease
Topline results from ALKIVIA study evaluating three myositis subsets (immune-mediated necrotizing myopathy (IMNM), anti-synthetase syndrome (ASyS) and dermatomyositis (DM)) expected in second half of 2026
Topline results from UNITY study (Sjögren’s disease) expected in 2027
Registrational study in Graves’ disease (GD) to initiate in first half of 2026, expanding development in thyroid-driven autoimmunity, including ongoing registrational studies in thyroid eye disease (TED)
Topline results from UplighTED studies expected in second half of 2026
Proof-of-concept studies ongoing in systemic sclerosis (SSc) and antibody mediated rejection (AMR)
Topline Phase 2 data from lupus nephritis do not support advancing to registrational study
Empasiprubart Development
Empasiprubart, a first-in-class, monoclonal antibody that specifically binds to C2, is currently being evaluated in three indications, including multifocal motor neuropathy (MMN), CIDP and delayed graft function (DGF).
Topline results from registrational EMPASSION study (MMN) expected in second half of 2026
Registrational EMVIGORATE and EMNERGIZE studies ongoing (CIDP)
Topline data from Phase 2 VARVARA study (DGF) expected around year-end 2025
Stopped development of empasiprubart in DM due to operational challenges with enrollment of proof-of-concept EMPACIFIC study
ARGX-119 Development
ARGX-119, a first-in-class agonist antibody that targets muscle-specific kinase (MuSK), is now a registrational asset following positive proof-of-concept data in congenital myasthenic syndromes (CMS). ARGX-119 is also being evaluated in amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA).
CMS registrational study on track to start in 2026
Phase 2a proof-of-concept study ongoing in ALS; topline results expected in first half of 2026
SMA proof-of-concept study on track to start by end of 2025
Advance four new pipeline molecules and generate sustainable value through continued investment in Immunology Innovation Program
argenx continues to invest in its Immunology Innovation Program (IIP) to drive long-term sustainable pipeline growth. Through the IIP, four new pipeline candidates have been nominated, including: ARGX-213, targeting FcRn and further solidifying argenx’s leadership in this biology; ARGX-121, a first-in-class molecule targeting IgA; ARGX-109, targeting IL-6, which plays an important role in inflammation, and a fourth pipeline candidate, a first-in-class sweeping antibody for which the target has not yet been disclosed.
Phase 1 study for ARGX-109 expected to complete by end of 2025; Phase 1 studies for ARGX-213 and ARGX-121 expected to complete in first half of 2026.
Q3 results and update, 30 October 2025
The naming process for new drugs is an interesting phenomenon.
A drug with a numeric code name receives its official, non-proprietary generic name when it is advanced into clinical trials, while its proprietary brand name is developed and approved during the FDA drug review and marketing stage.
The Naming Process
Early Development (Discovery and Preclinical Research): Initially, a potential drug compound is identified by a chemical name (describing its molecular structure) and a simple internal company code name, usually a series of letters and numbers (e.g., PF-04965842-01 for Pfizer). This allows the compound to be categorized and tracked internally.
Clinical Trials (Phase I, II, III): If the compound shows enough promise to enter human testing, the process for assigning a formal generic (non-proprietary) name begins.
The manufacturer submits proposed names to the United States Adopted Names (USAN) Council, which then works with the World Health Organization (WHO) International Nonproprietary Names (INN) expert group to select a single, universal name. This name is based on a stem system that helps healthcare professionals identify the drug’s class or action (e.g., names ending in “-tinib” are tyrosine kinase inhibitors).
Regulatory Review and Marketing (FDA Approval): As the drug progresses through clinical trials and the manufacturer plans for potential commercialization, the process for creating a brand (proprietary) name starts.
The pharmaceutical company develops a brand name, which must undergo extensive screening for safety, marketability, and linguistic checks across different languages.
The proposed brand name is then submitted to regulatory bodies like the FDA for approval. The FDA uses tools and simulations (including checking handwritten prescriptions) to ensure the name is not easily confused with any other existing drug names, which could lead to medication errors.
Once the brand name is approved by the relevant health authorities and the drug itself is approved for public use, it can be marketed under that name.
In essence, the generic name is for universal scientific and medical identification, while the brand name is for commercial marketing purposes after the drug has proven safe and effective.
AI overview, 15 November 2025
Another interesting topic is registrational assets, of which Argenx has a number. Argenx-119 has just become a registrational asset.
A registrational asset for a drug company is a drug candidate, intellectual property (such as patents), or related data that is in the late stages of development and intended for submission to regulatory authorities (like the FDA or EMA) to gain marketing approval.
Key characteristics and components:
Regulatory Focus: The primary goal of a registrational asset is to satisfy the stringent requirements for drug registration, which involves demonstrating the drug’s safety, efficacy, and quality through extensive research and clinical trials.
Late-Stage Development: These assets have usually progressed past early-stage research (which is generally expensed) into the development phase, where the technical and commercial feasibility of the product has been established, allowing development costs to be capitalised as an intangible asset on the balance sheet.
Future Economic Benefit: A key accounting criterion for capitalising a development asset is the probability that it will generate future economic benefits, such as future sales once approved and marketed.
Clinical Data: The asset includes the comprehensive data package, particularly from successful clinical trials, that forms the basis of a New Drug Application (NDA) or equivalent submission.
Valuation: The potential value of a registrational asset is a crucial factor in investment decisions and company valuations within the biopharma industry, often assessed using methods like risk-adjusted net present value (rNPV).
Strategic Importance: The identification and development of registrational pathways are a core part of a pharmaceutical company’s asset development strategy.
In essence, a registrational asset is an unapproved, but promising, drug product that is on the path to becoming a fully approved and commercially viable medicine.
AI Overview, 15 November 2025
We begin to see why ArgenxSE is such an exciting business.
argenx is a global immunology company committed to improving the lives of people suffering from severe autoimmune diseases. Partnering with leading academic researchers through its Immunology Innovation Program (IIP), argenx aims to translate immunology breakthroughs into a world-class portfolio of novel antibody-based medicines. argenx developed and is commercializing the first approved neonatal Fc receptor (FcRn) blocker and is evaluating its broad potential in multiple serious autoimmune diseases while advancing several earlier stage experimental medicines within its therapeutic franchises.
Argenx web site
This last quote, telling us what ArgenxSe is all about, says it all. Argenx is pioneering a new and extremely promising area of drug development, offering relief to many thousands of previously almost untreated patients.
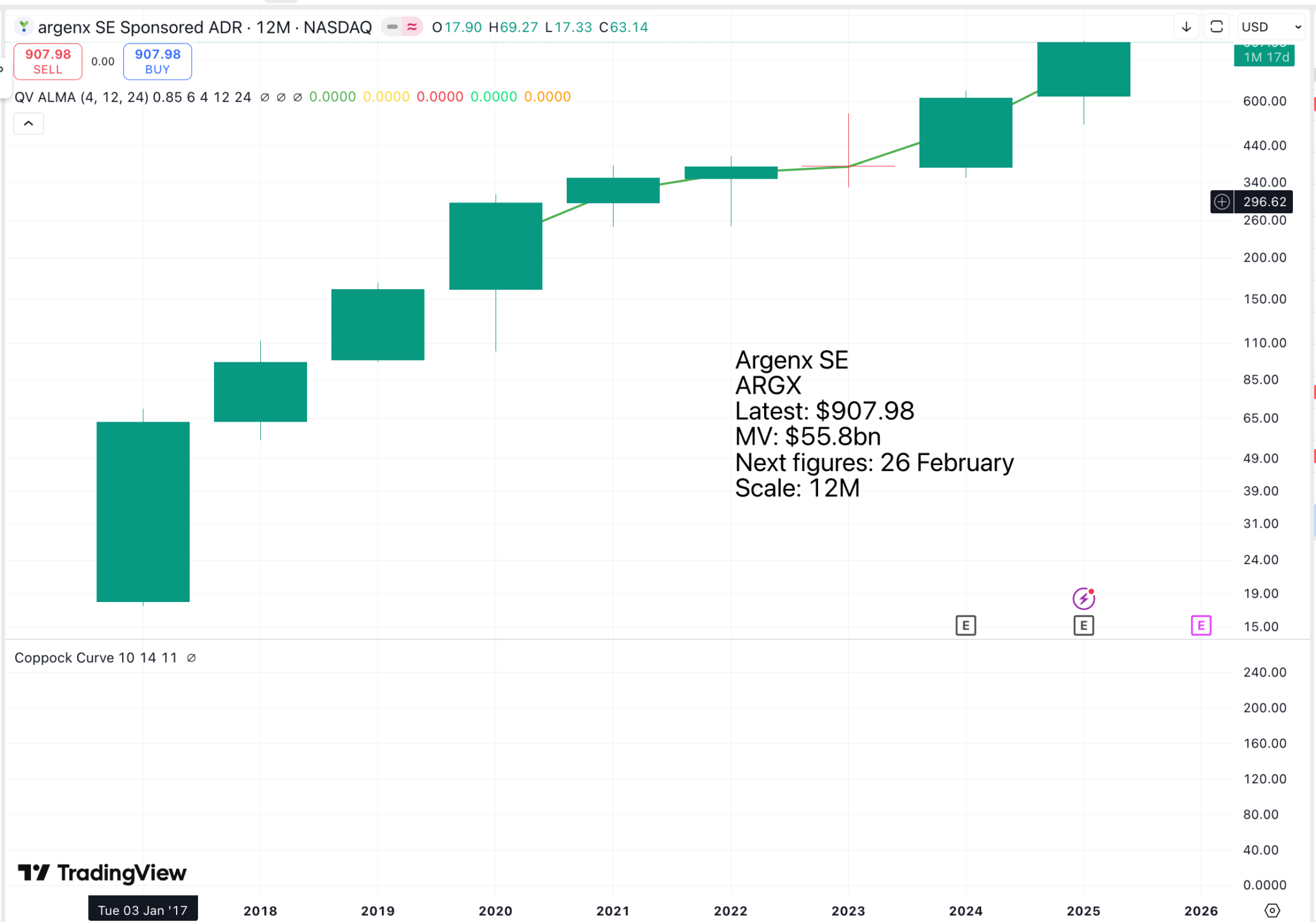
This potential, combined with the strong growth already being achieved, is driving the share price, which is steadily forging higher.
Share Recommendations
ArgenxSE. ARGX
Strategy – Keep Buying Argenx (ARGX)
I was going to write about a number of exciting drug development and healthcare companies in this alert because there is so much happening, but eventually I decided to focus on ArgenxSE. There is plenty to read, but I think it is worth it if you want to understand why ArgenxSE has such huge potential. There will be wins and losses along the way, but I believe that by 2030, ArgenxSE will be a much bigger company and still very exciting.
On 16 July 2024, ArgenxSE set out its Vision 2030 programme at a Research & Development Day.
Argenx SE formally unveiled its Vision 2030 during its R&D Day on July 16, 2024. The company had previously announced its plan to present this vision in a press release dated June 17, 2024.
The “Vision 2030” outlines argenx’s long-term goals to transform the treatment of autoimmune diseases, which include:
Reaching at least 50,000 patients globally with an argenx medicine.
Achieving 10 labeled indications across all approved assets, including their lead product VYVGART and potentially others.
Bringing five new molecules into Phase 3 development by 2030.
AI Overview, 15 November 2025
Since then, the share price has roughly doubled as the company has attracted a growing crowd of investors, who appreciate the impressive progress the company is making and the breakthroughs it is achieving for a growing number of patients, who need what Argenx is doing. In effect, the latest acceleration in the rate at which the shares are climbing is being driven by Vision 2030 and the progress the company is making towards realising its goals.



